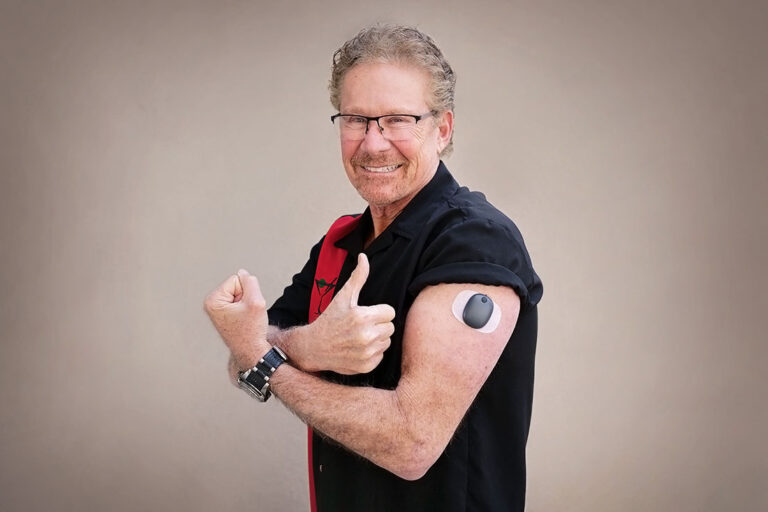
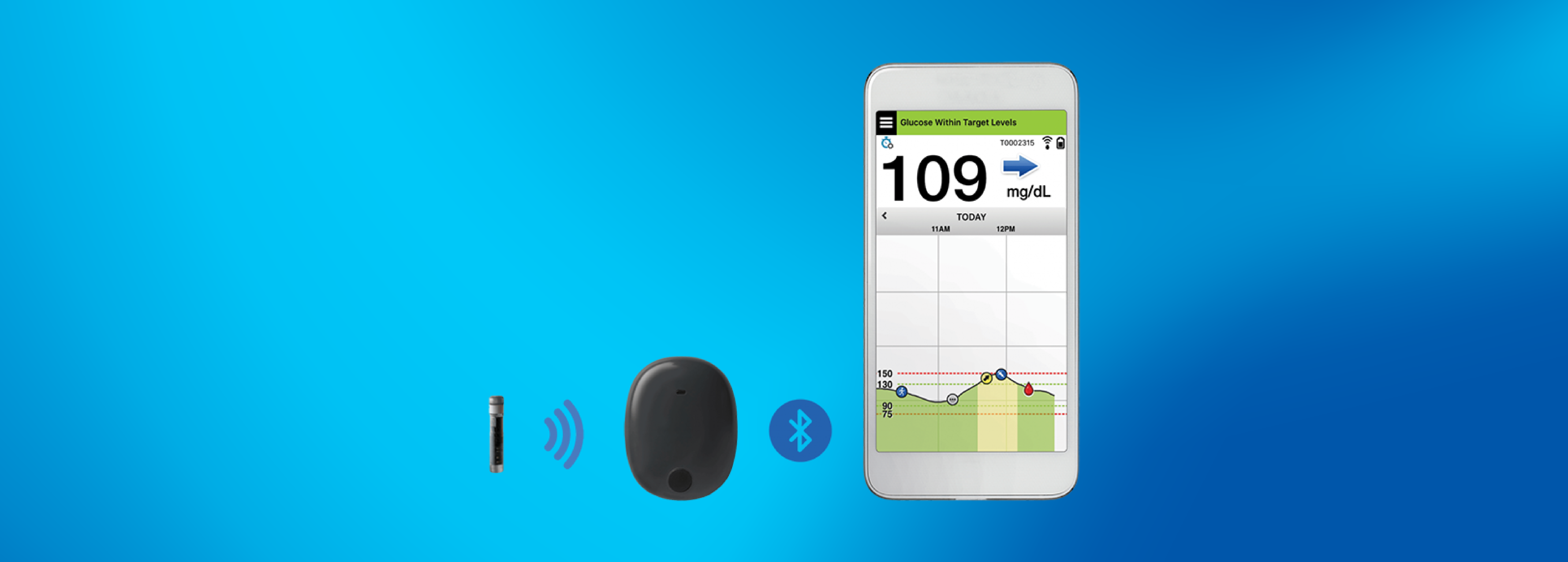
Eversense Implantable Glucometer Gets FDA Approval as Alternative to Finger Tests - 20451 Seneca Meadows Pkwy, Germantown, MD 20876, USA

Beyond Type 1 - NOW APPROVED BY THE FDA - a new CGM is on the scene! The Eversense from Senseonics is the first implantable continuous glucose monitor. It lasts 90 days
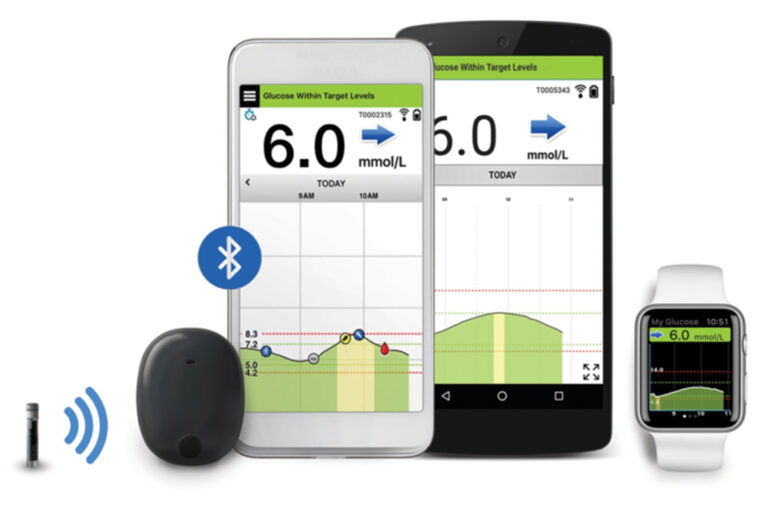
Eversense E3 CGM Approved for Two Sensors per Year: Your “Happily Ever(sense) After” - Taking Control Of Your Diabetes®

Senseonics Receives FDA Approval To Expand Eversense CGM Certification To Nurse Practitioners, PAs | Medical Product Outsourcing




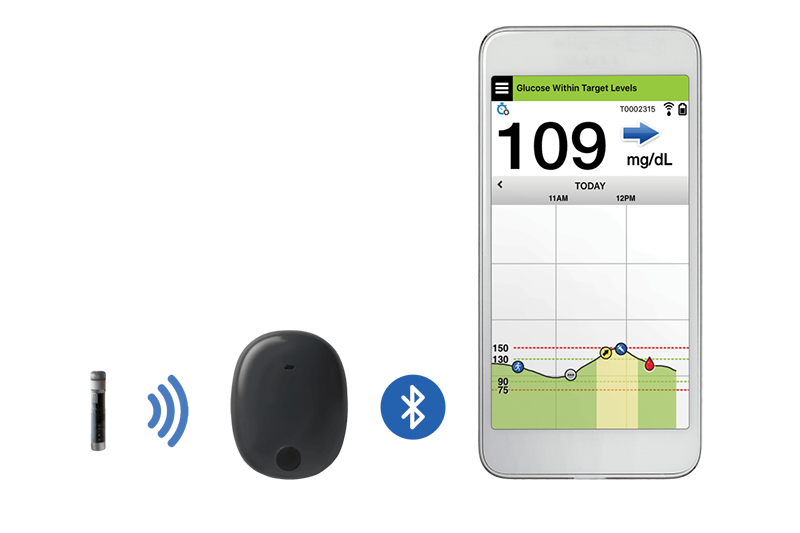










![6-Month Glucose Implant Sensor Receives CE Mark Approval [video]|Health Tech Insider 6-Month Glucose Implant Sensor Receives CE Mark Approval [video]|Health Tech Insider](https://i0.wp.com/healthtechinsider.com/wp-content/uploads/Eversense_CGM_with_video_600x275.jpg?fit=600%2C276&ssl=1)